Injectable for Profile Improvement & Double Chin
KYBELLA®
Improve the look of fuller chins with KYBELLA®, a non-surgical, non-invasive injectable for profile improvement & double chin.
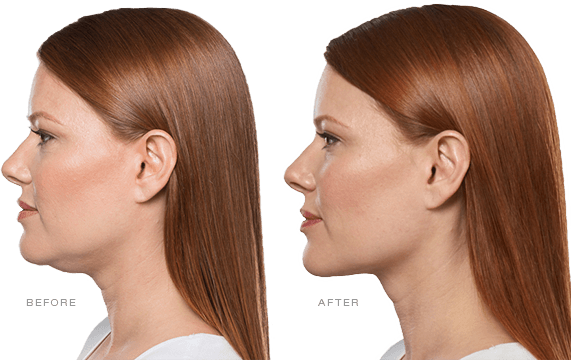
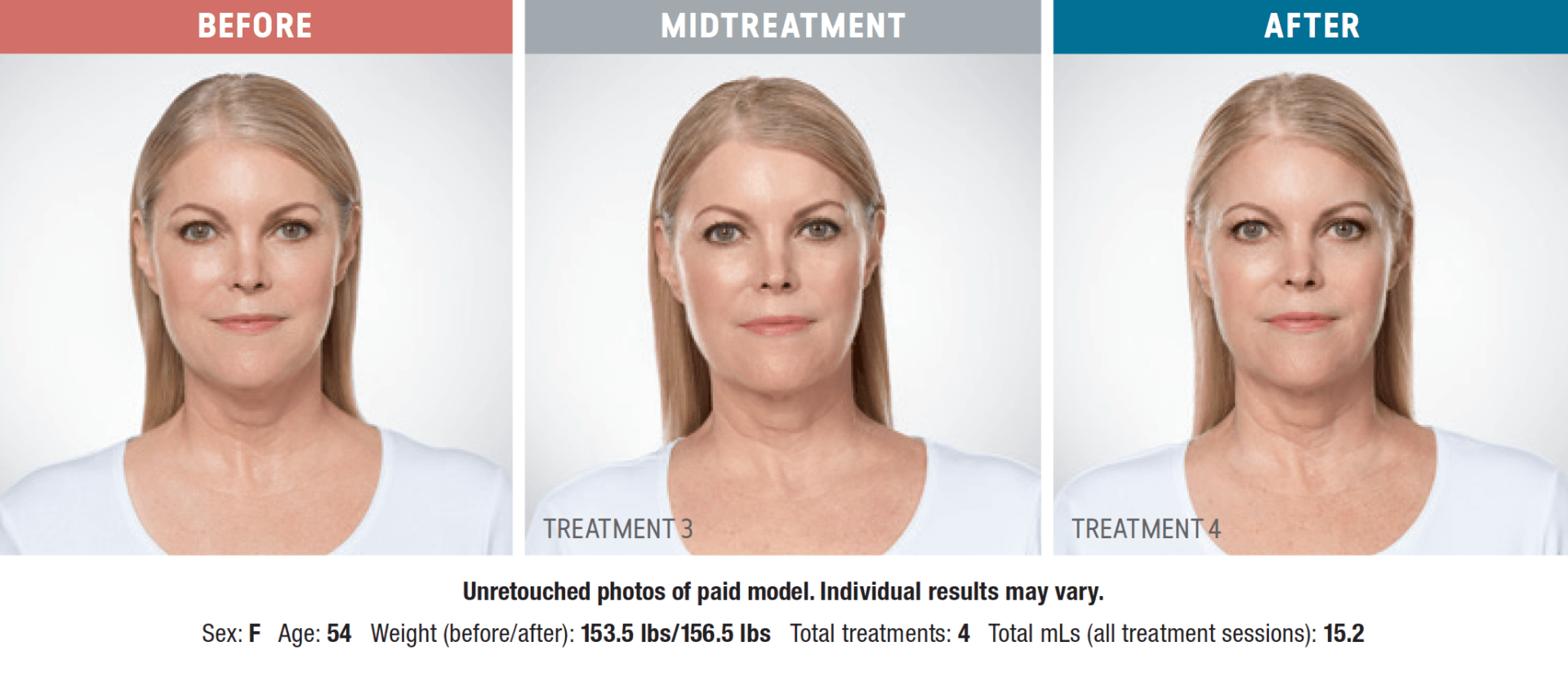
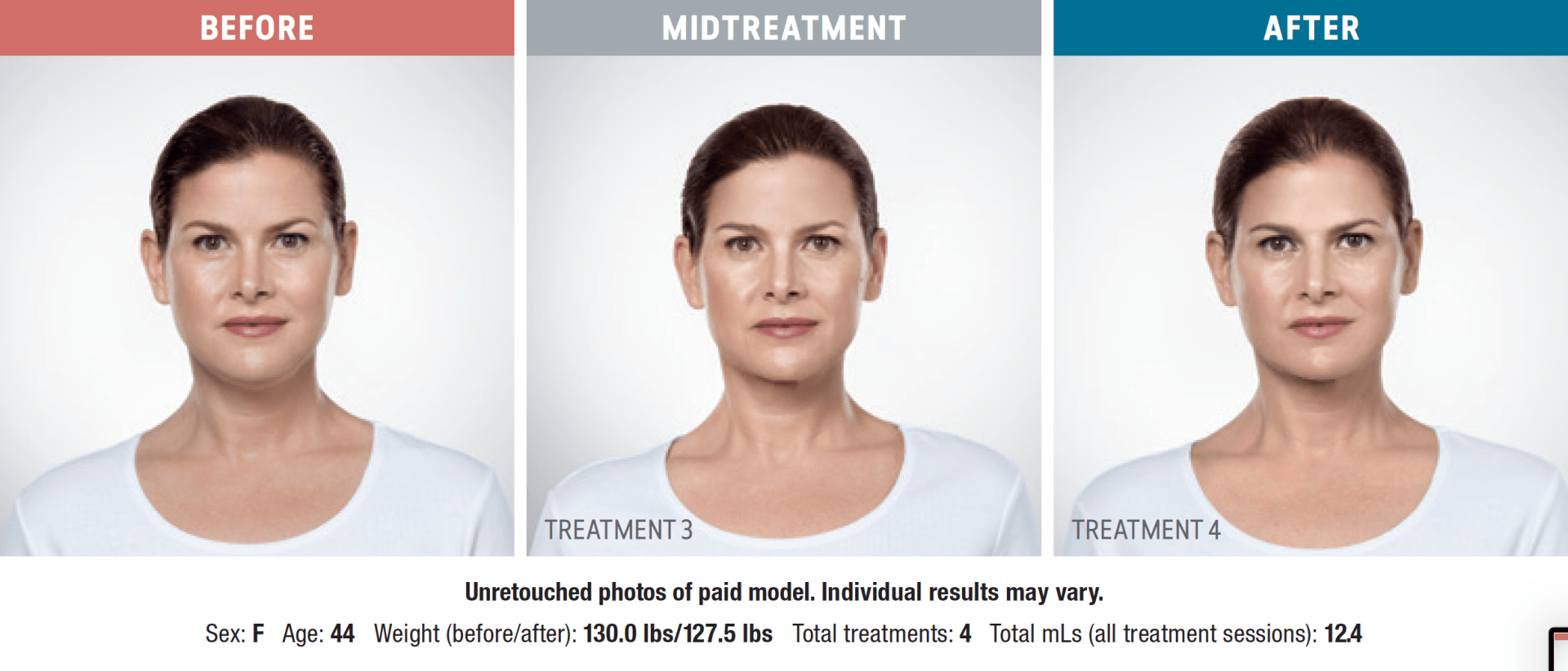
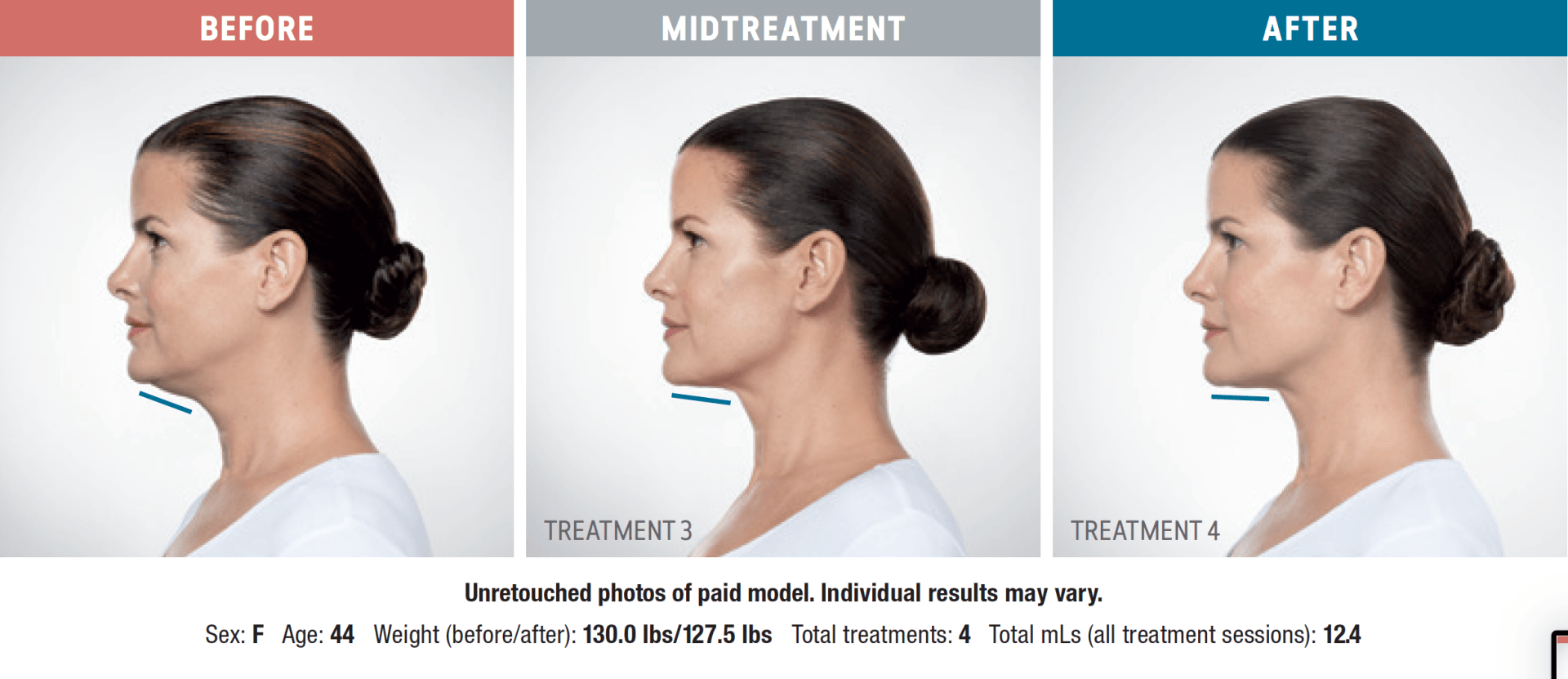
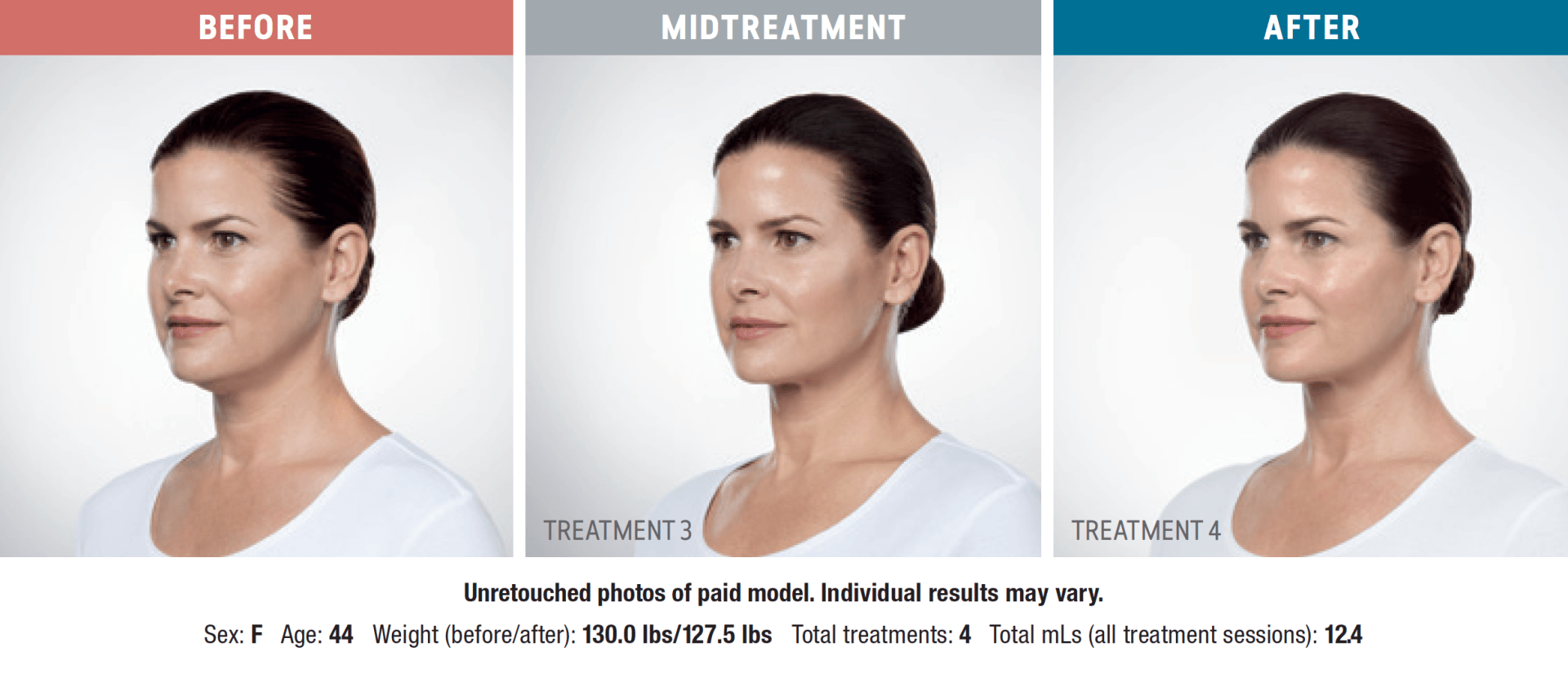
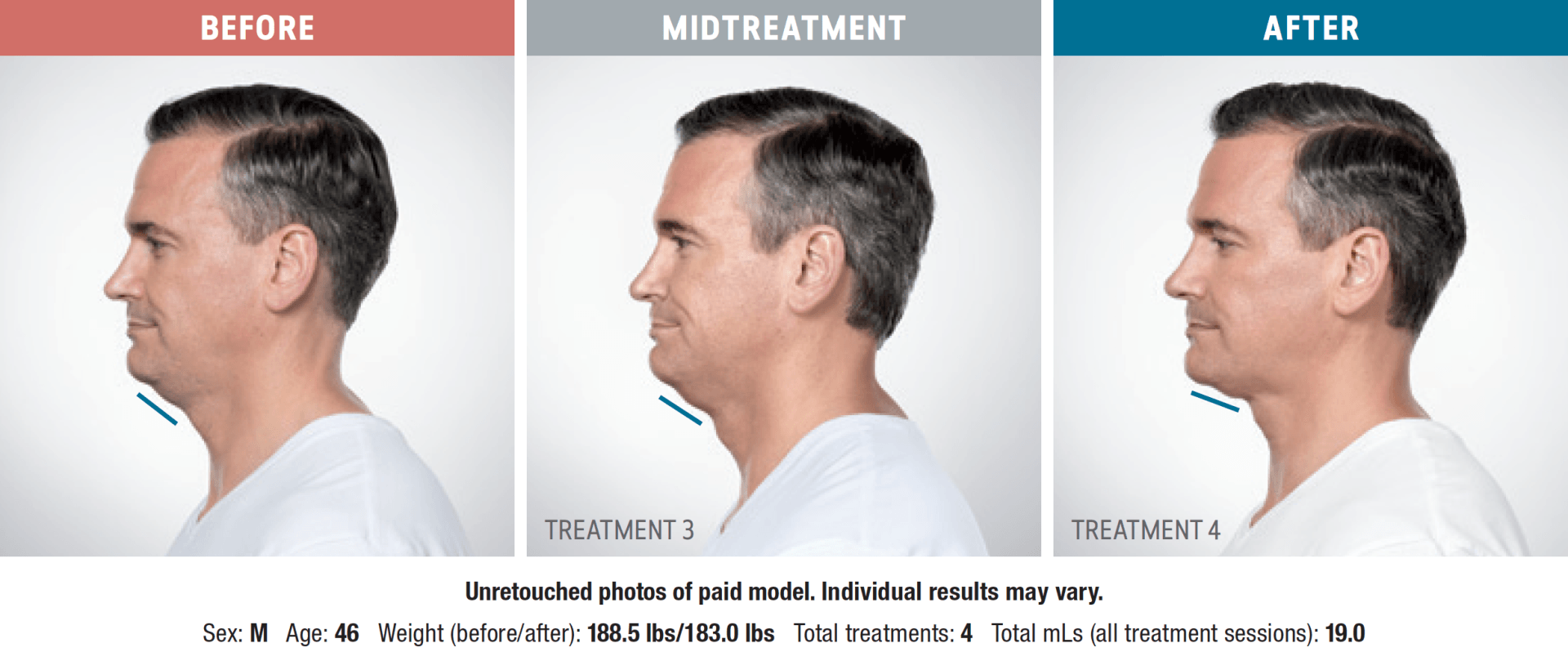
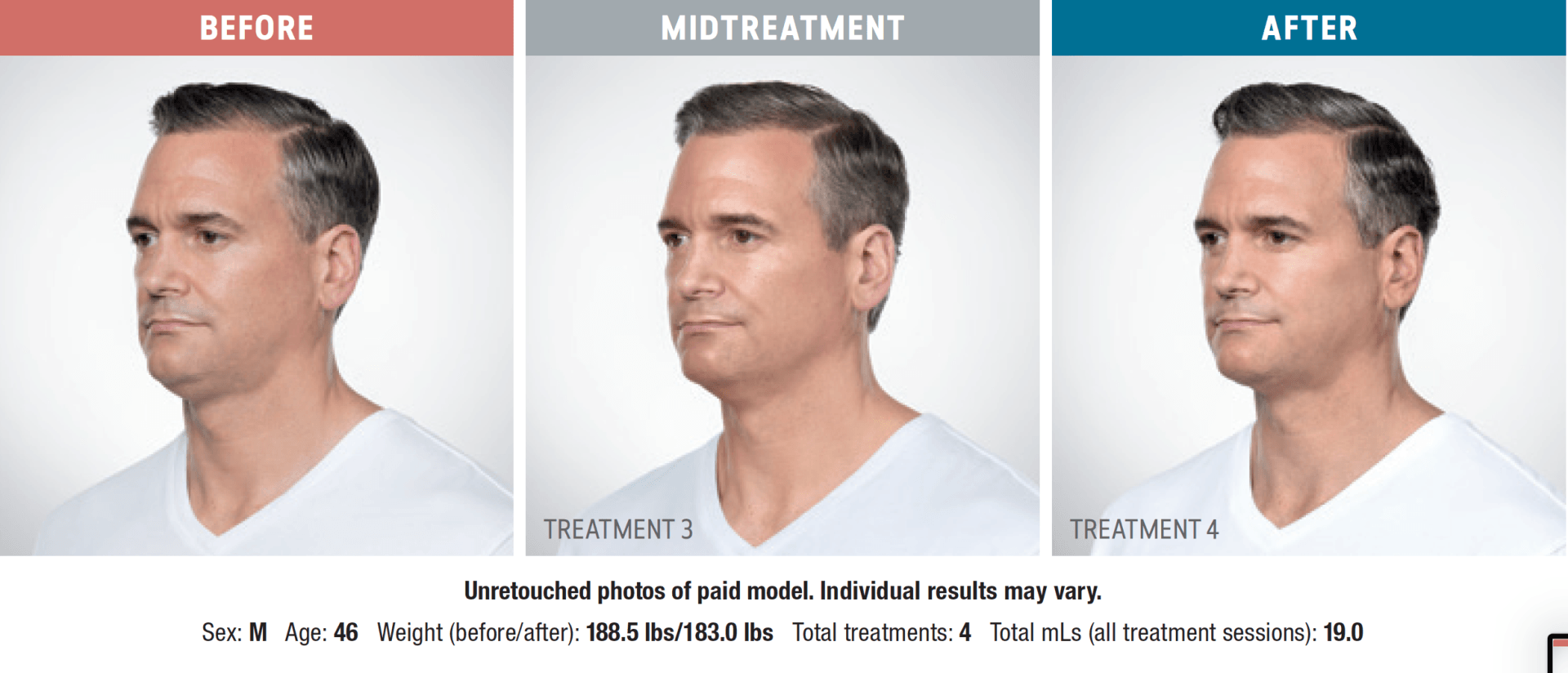
Have you noticed your chin profile sagging or sloping? This sagging—called submemental fullness—can be caused by aging, weight gain, or genetics. Targeting this area with diet and exercise alone is extremely difficult. Now you can reduce the appearance of a double chin with KYBELLA®, an injectable, non-surgical treatment for use on the fat below the chin.
KYBELLA® is the only FDA-approved injectable treatment that destroys fat cells in the treatment area under the chin to leave you with a sleeker chin profile. The aesthetic specialists at Integrity Health & Wellness will design a treatment plan tailored to your desired outcome.
KYBELLA® may be right for you if:
- You’re bothered (unhappy, self-conscious, embarrassed) by fat under the chin, also known as submental fullness
- You feel the condition makes you look older or heavier than you actually are
- You don’t want to have surgery
- You eat well and exercise, but submental fullness does not go away
Schedule a consultation today with our aesthetic specialists to learn more!
Earn Points on Kybella® & more with Allē Rewards!
Did you know that you could be earning points on purchases of BOTOX® Cosmetic, Juvéderm®, and Kybella®? With the new Allē loyalty program (formerly known as Brilliant Distinctions™), you can earn points on your favorite Allergan Aesthetics® brands and a variety of other in-office treatments. Join today to start enjoying rewards, and so much more!